Metal Control Solutions for the Pharmaceuticals, Nutraceuticals, and Cosmetics Industries
These industries require exceptional levels of purity, sanitation, and regulatory compliance. Metal contamination from ingredient sourcing, mechanical wear, or cross-contamination during changeovers can seriously affect product safety, compliance with cGMP/ICH/FDA standards, and brand trust.
MPI’s magnetic separation and inspection solutions are engineered for sanitary, validated production environments, helping prevent:
- Contamination in powders, granules, liquids, and gels
- Equipment damage in high-precision mixers, mills, and packaging systems
- Product recalls due to metal fragments or foreign objects
MPI’s sanitary-grade magnetic separation and detection technologies support high-spec, high-purity production of medications, supplements, personal care items, and beauty products. With solutions tailored for:
- Hygienic design and documentation
- High-value product protection
- Regulatory and audit compliance
- Process integrity from raw to final product
MPI ensures manufacturers meet the highest global standards, without compromising throughput or cleanliness.
Where MPI solutions are used: ingredient intake, blending, compounding, processing, packaging, and final QA steps.
| Process Stage | MPI Product | Purpose |
|---|---|---|
| Raw ingredient intake | Drawer or pneumatic magnet | Capture contamination from incoming API or excipients |
| Blending and mixing | liquid trap magnet | Ensure uniform, contaminant-free mixing of active compounds |
| Tablet/capsule filling | Drawer or metal detector | Protect mechanical components and ensure part quality |
| cream/gel processing | Inline drawer and plate | Remove contaminants from emulsions or suspensions |
| Final packaging QA | Metal detector or X-ray | Verify product integrity before shipment |
Turnkey Solutions for Pharmaceutical, Nutraceutical, and Cosmetics Manufacturers From Magnetic Products, Inc
-
Ensures Compliance with cGMP, FDA, and ICH Guidelines
MPI systems are built to meet or exceed strict pharmaceutical-grade requirements with polished stainless steel, sanitary welds, and validated cleaning.
-
Protects Sensitive Processing Equipment
Captures fine metal before it damages mills, capsule fillers, fluid bed dryers, or mixers, minimizing costly repairs and production downtime.
-
Removes Fine and Weakly Magnetic Particles
Rare-earth magnetic cartridges remove even sub-micron particles that may be missed by sieves or filters—ideal for high-purity powders.
-
Tool-Free and Clean-In-Place Designs Available
MPI systems offer quick-clean and self-clean options for frequent product changeovers and validated cleaning processes.
-
Compatible with Dry, Liquid, and Slurry-Based Materials
Customizable for powders, pills, tablets, serums, creams, gels, or active ingredient suspensions across various viscosities.
-
Supports Allergen Control and Cross-Contamination Prevention
Fast, efficient cleaning reduces risk between batches, supporting strict hygiene requirements and multi-product facilities.
-
Audit-Ready and Documentation Support
MPI systems include support for material certifications, weld maps, inspection protocols, and installation qualification (IQ)/operational qualification (OQ) readiness.
-
Compact, High-Throughput Designs for Cleanrooms
MPI units are designed for limited-space environments and fit cleanroom installations while maintaining high flow rates and repeatable performance.
How Do Pharmaceutical, Nutraceutical, and Cosmetics Manufacturers Use Magnets?

| MPI PRODUCT TYPE | WHERE | WHY | WHEN | HOW |
|---|---|---|---|---|
| Drawer Magnets (Manual-Clean, Quick-Clean, Self-Clean) | Gravity-fed feed hoppers, tablet presses, dry blenders | Gentle on ingredients, tool-free access, and suitable for fine materials. | Before mixing, encapsulation, tableting, or packaging | Capture fine ferrous contamination in powders and granules |
| Pneumatic Line Drawer Magnets (Quick-Clean, Self-Clean) | In-line vacuum or pressure conveying of bulk powder | Removes contaminants during ingredient transfer. Maintains purity and protects downstream equipment. | From central material feed systems to processing lines | Magnetic cartridges inside sealed housings pull metal from high-speed flows |
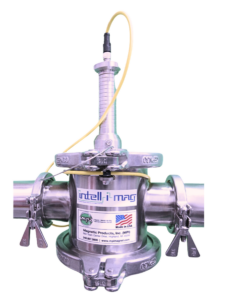
| Liquid Trap Magnets (Sanitary, Quick-Clean, Self-Clean) | Pipelines transporting creams, gels, suspensions, and solutions | Capture tramp metal in viscous or aqueous materials. Maintains system sterility and prevents formulation issues. | Before mixing, homogenizing, or packaging | Rare-earth magnets within sealed housings attract contaminants as fluid flows |
| Inline Drawer and Plate Magnets | Vertical or space-constrained sanitary lines | Fits into tight piping configurations common in cleanroom facilities. Low turbulence and optimized magnetic coverage. | During bulk tank transfer or post-processing | Angled magnetic design enables consistent contact with high-viscosity products |
| Metal Detectors (Conveyor or Pipeline Style) | Final inspection before bottling, blister packaging, or carton loading | Detects all metal types, including stainless steel fragments. Ensures regulatory compliance and batch integrity. | Last check before the product leaves the controlled manufacturing area | Electromagnetic sensing zones detect metal, triggering reject mechanisms |
| X-Ray Inspection Systems (Packaged Products) | Post-packaging for tablets, pouches, vials, or boxes | Detects non-metallic contaminants like glass, rubber, bone. Protects against recalls and enhances consumer safety. | Final QA checkpoint before secondary packaging | High-resolution imaging identifies irregular densities |